Research & Development
The BioThymus technology is based on the discovery and characterisation of multipotent thymic stem cells capable of deriving the key thymic epithelial cells (TECs) that control thymopoiesis (T cell development) and central tolerance (education of T cells). This creates populations of T cells that circulate our body to fight pathogens such as viruses, bacteria and cancers and that are also self-tolerant.
Bioengineered Thymus (BioThymus)
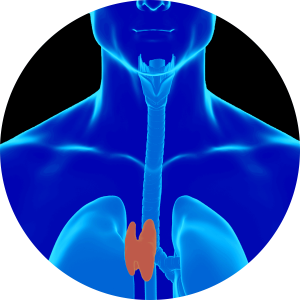
This preclinical programme is focussed on creation of a bioengineered thymus (BioThymus). The thymus is a small organ, located in front of the heart. In recent years the critical function of the thymus has become better understood, and its importance in development of life-long protection against infections, malignancy and the recognition of ‘self’ are just some of its essential functions.
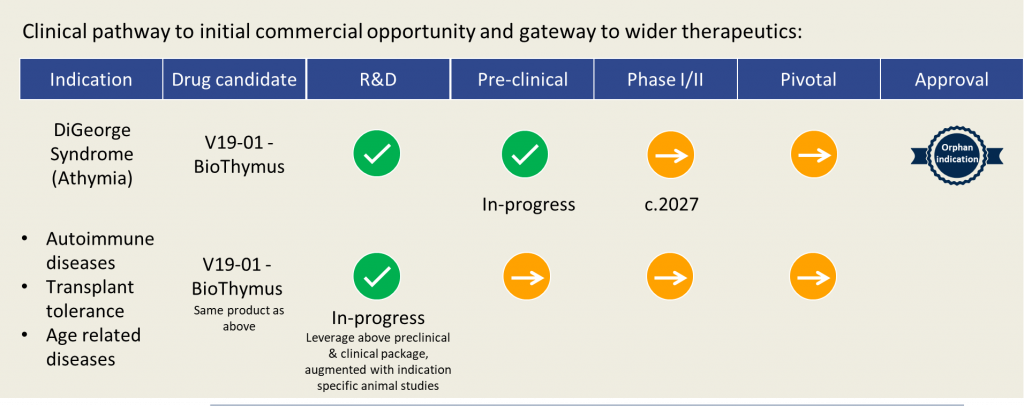
In rare cases, infants can be born without a thymus, a condition known as complete DiGeorge Syndrome. These athymic infants are significantly immunocompromised and without treatment, may die within 2 to 3 years.
Pioneering scientific research, has established a ground-breaking technology to build a BioThymus. This research has shown that the BioThymus has the potential to provide the immune system functions that are lacking in athymic infants.
Immune system reprogramming – In vivo proof of concept
In vivo proof of concept of immune reconstitution was achieved following implantation of the BioThymus into an athymic humanised immunodeficient mouse model. The data demonstrated upregulation of critical gene expression, thymocyte education, establishment of central tolerance and generation of circulating mature human T cells, and provides the basis for a therapeutic approach to rejuvenation of adaptive immunity.
Development towards clinical trial
Clonogenic multipotent thymic epithelial cells, capable of long-term expansion, are able to reconstitute attributes of the native thymus when combined with thymic interstitial cells and the 3-dimensional BioThymus scaffold. Cells are seeded onto the scaffold using an aseptic procedure and after subsequent culture, the final product will be provided to the clinician in a self-contained transport bioreactor. The bioreactor ensures the appropriate environment is maintained during transport to the hospital, maintaining quality, sterility and viability of the product. Aspects of the manufacturing process will be optimised during GMP translation to allow for simpler, robust and scalable processes capable of commercialisation.
Videregen is working to translate the technology into a GMP ready manufacturing process in preparation for safety/toxicology testing to support a future clinical trial of the BioThymus in complete DiGeorge Syndrome.
Future applications of the therapeutic platform
The potential clinical and therapeutic applications of the BioThymus platform are many and highly significant; such as primary immune-deficiencies, tolerance to organ transplantation and auto-immunity (for example Myasthenia gravis, rheumatoid arthritis and Type I diabetes), which afflict significant patient populations.
In addition, there is growing evidence that ageing (i.e. immune deterioration with age) is related to lack of thymus function in later life, leading to immune dysfunction and onset of age related damage and disease due to aberrant T cell conditioning. Restoration of thymus function could have profound implications in this area.
References
- Campinoti et al. 2020. Reconstitution of a functional human thymus by postnatal stromal progenitor cells and natural whole-organ scaffolds. Nature Communications, 11:6372 https://doi.org/10.1038/s41467-020-20082-7
- Ragazzini et al., 2023. Defining the identity and the niches of epithelial stem cells with highly pleiotropic multilineage potency in the human thymus. Developmental Cell 58, 1–19. https://doi.org/10.1016/j.devcel.2023.08.017